Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 Cs fallout
Cs falloutOta, Masakazu; Koarashi, Jun
Science of the Total Environment, 816, p.151587_1 - 151587_21, 2022/04
Times Cited Count:7 Percentile:68.71(Environmental Sciences)In forests affected by the Fukushima Daiichi Nuclear Power Plant accident, trees became contaminated with  Cs. However,
Cs. However,  Cs transfer processes determining tree contamination (particularly for stem wood, which is a prominent commercial resource in Fukushima) remain insufficiently understood. This study proposes a model for simulating the dynamic behavior of
Cs transfer processes determining tree contamination (particularly for stem wood, which is a prominent commercial resource in Fukushima) remain insufficiently understood. This study proposes a model for simulating the dynamic behavior of  Cs in a forest tree-litter-soil system and applied it to two contaminated forests (cedar plantation and natural oak stand) in Fukushima. The model-calculated results and inter-comparison of the results with measurements elucidated the relative impact of distinct
Cs in a forest tree-litter-soil system and applied it to two contaminated forests (cedar plantation and natural oak stand) in Fukushima. The model-calculated results and inter-comparison of the results with measurements elucidated the relative impact of distinct  Cs transfer processes determining tree contamination. The transfer of
Cs transfer processes determining tree contamination. The transfer of  Cs to trees occurred mostly (
Cs to trees occurred mostly ( 99%) through surface uptake of
99%) through surface uptake of  Cs directly trapped by leaves or needles and bark during the fallout. By contrast, root uptake of
Cs directly trapped by leaves or needles and bark during the fallout. By contrast, root uptake of  Cs from the soil was unsubstantial and several orders of magnitude lower than the surface uptake over a 50-year period following the accident. As a result, the internal contamination of the trees proceeded through an enduring recycling (translocation) of
Cs from the soil was unsubstantial and several orders of magnitude lower than the surface uptake over a 50-year period following the accident. As a result, the internal contamination of the trees proceeded through an enduring recycling (translocation) of  Cs absorbed on the tree surface at the time of the accident. A significant surface uptake of
Cs absorbed on the tree surface at the time of the accident. A significant surface uptake of  Cs at the bark was identified, contributing 100% (leafless oak tree) and 30% (foliated cedar tree; the remaining surface uptake occurred at the needles) of the total
Cs at the bark was identified, contributing 100% (leafless oak tree) and 30% (foliated cedar tree; the remaining surface uptake occurred at the needles) of the total  Cs uptake by trees. It was suggested that the trees growing at the study sites are currently (as of 2021) in a decontamination phase; the activity concentration of
Cs uptake by trees. It was suggested that the trees growing at the study sites are currently (as of 2021) in a decontamination phase; the activity concentration of  Cs in the stem wood decreases by 3% per year, mainly through radioactive decay of
Cs in the stem wood decreases by 3% per year, mainly through radioactive decay of  Cs and partly through a dilution effect from tree growth.
Cs and partly through a dilution effect from tree growth.
Ota, Masakazu; Kwamena, N.-O. A.*; Mihok, S.*; Korolevych, V.*
Journal of Environmental Radioactivity, 178-179, p.212 - 231, 2017/11
Times Cited Count:14 Percentile:43.57(Environmental Sciences)Environmental transfer models assume that organically-bound tritium (OBT) is formed directly from tissue-free water tritium (TFWT) in environmental compartments. Nevertheless, studies in the literature have shown that measured OBT/TFWT ratios are variable. The importance of soil-to-leaf HTO transfer pathway in controlling the leaf tritium dynamics is not well understood. A model inter-comparison of two tritium transfer models (CTEM-CLASS-TT and SOLVEG-II) was carried out with measured environmental samples from an experimental garden plot set up next to a tritium-processing facility. The garden plot received one of three different irrigation treatments - no external irrigation, irrigation with low tritium water and irrigation with high tritium water. The contrast between the results obtained with the different irrigation treatments provided insights into the impact of soil-to-leaf HTO transfer on the leaf tritium dynamics. Concentrations of TFWT and OBT in the garden plots that were not irrigated or irrigated with low tritium water were variable, responding to the arrival of the HTO-plume from the tritium-processing facility. In contrast, for the plants irrigated with high tritium water, the TFWT concentration remained elevated due to a continuous source of high HTO in the soil. Calculated concentrations of OBT in the leaves showed an initial increase followed by quasi-equilibration with the TFWT concentration. In this quasi-equilibrium state, concentrations of OBT remained elevated and unchanged despite the arrivals of the plume. These results from the model inter-comparison demonstrate that soil-to-leaf HTO transfer significantly affects OBT/TFWT ratio in the leaf regardless of the atmospheric HTO concentration, only if there is elevated HTO concentrations in the soil. The results of this work indicate that assessment models should be refined to consider the importance of soil-to-leaf HTO transfer to ensure that dose estimates are accurate and conservative.
Irisawa, Keita; Meguro, Yoshihiro
Journal of Nuclear Science and Technology, 54(3), p.365 - 372, 2017/03
Times Cited Count:2 Percentile:19.65(Nuclear Science & Technology) Au surfaces with a hyperthermal O
Au surfaces with a hyperthermal O molecular beam
molecular beamOkada, Michio*; Moritani, Kosuke; Fukuyama, Tetsuya*; Mizutani, Hironori*; Yoshigoe, Akitaka; Teraoka, Yuden; Kasai, Toshio*
Surface Science, 600(18), p.4228 - 4232, 2006/09
Times Cited Count:20 Percentile:64.46(Chemistry, Physical)Dissociative adsorption of hyperthermal O molecules on Cu
molecules on Cu Au(100) was investigated by X-ray photoemission spectroscopy in conjunction with a synchrotron light source. Comparing the O-uptake curve on Cu
Au(100) was investigated by X-ray photoemission spectroscopy in conjunction with a synchrotron light source. Comparing the O-uptake curve on Cu Au with that on Cu, it was found that the dissociative adsorption of O
Au with that on Cu, it was found that the dissociative adsorption of O is more activated (less reactive) due to Au alloying. The low-energy electron-diffraction (LEED) pattern of c(2
is more activated (less reactive) due to Au alloying. The low-energy electron-diffraction (LEED) pattern of c(2 2) for the clean surface turned into the (1
2) for the clean surface turned into the (1 1) LEED pattern during the oxidation by hyperthermal O
1) LEED pattern during the oxidation by hyperthermal O molecular beam, suggesting that the O adsorption induces the Cu-atom segregation on the surface.
molecular beam, suggesting that the O adsorption induces the Cu-atom segregation on the surface.
Kozai, Naofumi; Onuki, Toshihiko; Komarneni, S.*; Kamiya, Tomihiro; Sakai, Takuro; Oikawa, Masakazu*; Sato, Takahiro
Nuclear Instruments and Methods in Physics Research B, 210, p.513 - 518, 2003/09
Times Cited Count:8 Percentile:50.24(Instruments & Instrumentation)This study examined removal of cadmium from solution by two materials with high sorptivity for cadmium: a synthetic mica named Na-4 mica and an apatite. At an initial Cd concentration of 1 10
10 M and a starting pH of 3, the distribution coefficient of Na-4 mica for Cd (8.4
M and a starting pH of 3, the distribution coefficient of Na-4 mica for Cd (8.4 10
10 ml/g) was one order of mgnitude of higher than that of apatite (8.2
ml/g) was one order of mgnitude of higher than that of apatite (8.2 10
10 ml/g). On the other thand, micro-PIXE anaysis for a mixture of equal mass of Na-4 mica and apatite contacted with Cd solution under the same conditions revealed that Cd was selectively taken up by the apatite in the mixture, while Cd was not detected on the Na-4 mica in the mixture. This result by micro-PIXE analysis is not what is expected from the above distribution coefficient values of Na-4 mica and apatite. This result can be explained by irreversibility of the uptake and the difference in kinetics of the uptake by the Na-4 mica and apatite.
ml/g). On the other thand, micro-PIXE anaysis for a mixture of equal mass of Na-4 mica and apatite contacted with Cd solution under the same conditions revealed that Cd was selectively taken up by the apatite in the mixture, while Cd was not detected on the Na-4 mica in the mixture. This result by micro-PIXE analysis is not what is expected from the above distribution coefficient values of Na-4 mica and apatite. This result can be explained by irreversibility of the uptake and the difference in kinetics of the uptake by the Na-4 mica and apatite.
Kozai, Naofumi; Onuki, Toshihiko; Komarneni, S.*
Journal of Materials Chemistry, 17(12), p.2993 - 2996, 2002/12
Here we report the extremely high and selective uptake of selenium oxyanions by a novel exchanger, Ni Zn
Zn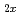 (OH)
(OH) (OCOCH
(OCOCH )
)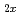 ・nH
・nH O that has brucite-type hydroxide layers with OCOCH
O that has brucite-type hydroxide layers with OCOCH anions in the interlayers. This Ni-Zn basic salt exhibited very high selectivity for Se(IV) (Kd = 9.0x10
anions in the interlayers. This Ni-Zn basic salt exhibited very high selectivity for Se(IV) (Kd = 9.0x10 ml/g with an initial Se(IV) concentration of 1x10
ml/g with an initial Se(IV) concentration of 1x10 M) in the presence of 0.1M Cl
M) in the presence of 0.1M Cl solution while the well known anionic clay, Mg
solution while the well known anionic clay, Mg Al(OH)
Al(OH) NO
NO ・nH
・nH O showed a Kd of 6.0x10
O showed a Kd of 6.0x10 ml/g under the same conditions. The uptake of Se(IV) on the Ni-Zn basic salt was found to be irreversible when treated with solutions containing 1N Cl
ml/g under the same conditions. The uptake of Se(IV) on the Ni-Zn basic salt was found to be irreversible when treated with solutions containing 1N Cl , 1N NO
, 1N NO
 , or 1N PO
, or 1N PO
 , while the Se(IV) sorbed on anionic clay was easily desorbed in a 1M Cl
, while the Se(IV) sorbed on anionic clay was easily desorbed in a 1M Cl solution. This novel exchanger also showed high Kd (2.6
solution. This novel exchanger also showed high Kd (2.6 10
10 ml/g at an initial Se concentration of 1
ml/g at an initial Se concentration of 1 10
10 M) for Se(VI) and therefore it is expected to be useful for decontamination and removal of selenium oxyanions from contaminated water.
M) for Se(VI) and therefore it is expected to be useful for decontamination and removal of selenium oxyanions from contaminated water.
Komarneni, S.*; Kozai, Naofumi; Paulus, W. J.*
Nature, 410(6830), P. 771, 2001/04
A radioactive legacy of the nuclear industry threatens many areas of the world with radioactive radium contamination from the solid and liquid waste left after extracting U from ores. Here we report that a synthetic clay of high charge is not only extremely selective for the uptake of Ra but also useful for Ra immobilization at room temperature. These results are of relevance for decontaminating the environmental and drinking water of Ra.
Furukawa, Jun*; Nakanishi, Tomoko*; Matsubayashi, Masahito
Nuclear Instruments and Methods in Physics Research A, 424(1), p.116 - 121, 1999/00
Times Cited Count:19 Percentile:77.26(Instruments & Instrumentation)no abstracts in English
; H.Gnaser*; W.O.Hofer*
Nuclear Instruments and Methods in Physics Research B, 28, p.540 - 547, 1987/00
Times Cited Count:11 Percentile:76.84(Instruments & Instrumentation)no abstracts in English
 transport in relation to root uptake of CO
transport in relation to root uptake of CO in rice plant
in rice plant*; ;
Soil Science and Plant Nutrition, 30(2), p.125 - 136, 1984/00
Times Cited Count:35 Percentile:84.15(Plant Sciences)no abstracts in English
E.A.Hegazy*; ; A.Rabie*; A.M.Dessouki*;
J.Appl.Polym.Sci., 26, p.3871 - 3883, 1981/00
Times Cited Count:56 Percentile:92.3(Polymer Science)no abstracts in English

 Zn and the concentration factor on Zn in marin organisms, 1; Uptake of
Zn and the concentration factor on Zn in marin organisms, 1; Uptake of 
 Zn in marine organisms
Zn in marine organisms; *; *
Journal of Radiation Research, 9(2), p.50 - 62, 1968/00
no abstracts in English